artplac
University of Twente professors Jutta Arens (Engineering Organ Support Technologies, Faculty of Engineering Technology) and Dimitrios Stamatialis (Advanced Organ Bioengineering and Therapeutics, Faculty of Science and Technology) are working on the artificial placenta system, called ArtPlac, together with partners from academia and industry from the Netherlands, Germany, Sweden, Ireland and Canada. The consortium, coordinated by neonatologists from Nuremberg, Germany, just received four million Euros from the EIC Pathfinder programme for the ArtPlac project which will last four years.
‘Every year, about two million neonatal deaths occur worldwide,’ says professor Arens. ‘Out of all the cases, 458 happen in the Netherlands. This might not sound like a large number, but it means 458 grieving families each year. Many of these newborns die due to lung and kidney failure, because they are born prematurely and their lungs aren’t developed yet.’
Invasive treatment
The current course of treatment for critically ill newborns is far from ideal, describes the scientist. After (premature) birth, a baby is assessed and either discharged or admitted for supervision. In some cases, the baby needs to be placed in an incubator and connected to a mechanical ventilator or even an artificial lung and a dialysis machine. ‘The mechanical ventilator works with pressure, which further damages premature lung tissue. This can lead to serious life-long health issues,’ Arens continues to explain why an artificial placenta is needed. ‘Moreover, the baby is connected to so many machines, it needs to be kept sedated. The treatment is very invasive and costly, but it is the best we have at the moment.’
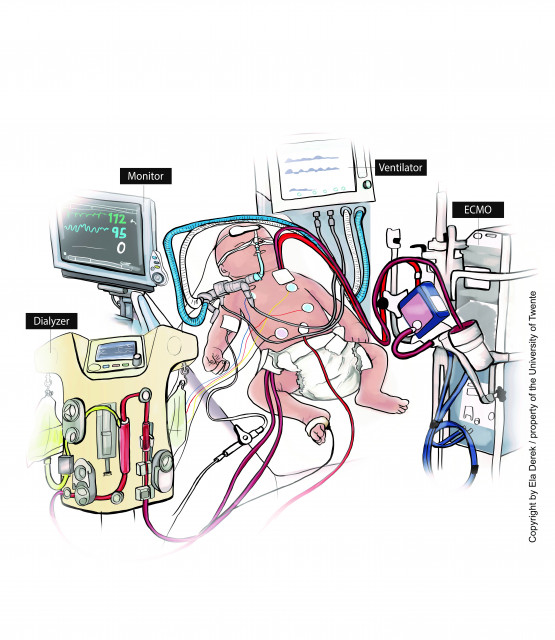
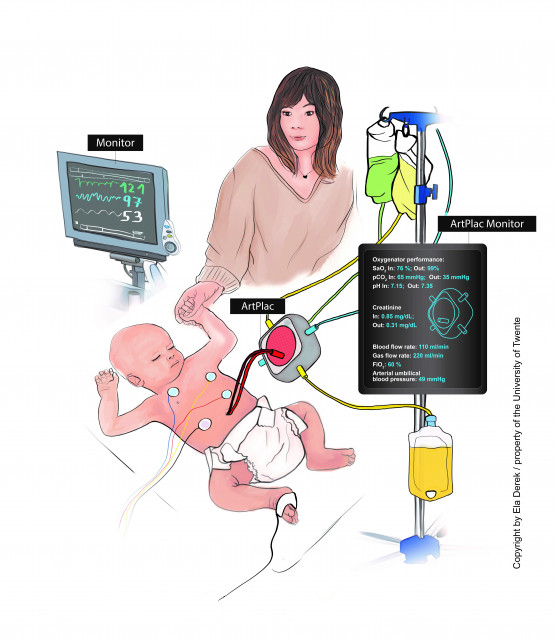
The illustrations showcasing the current treatment (on the left) and the artificial placenta system (on the right). Graphics by Ela Derek
The UT scientists’ goal is to replace the separate mechanical ventilator and the dialysis machine and develop an artificial placenta - one combined device for lung and kidney support which would be connected to the baby’s belly button. ‘This would result in much less invasive treatment provided to an awake and responsive baby,’ says professor Stamatialis. ‘With an artificial placenta, the baby doesn’t need to be sedated and it can interact with the family. This is very important – the impact on parents is a big part of this issue.’
‘With an artificial placenta, the baby doesn’t need to be sedated and it can interact with the family'
Artificial womb
Developing an artificial placenta isn’t the only approach that is being explored. Other researchers are also working on an artificial womb, a device that essentially allows the pregnancy to continue outside the mother’s body. ‘We believe our approach is gentler and safer for the baby,’ says Arens. ‘In an artificial womb the premature baby is kept submerged during and after birth in fluid and is connected to an artificial placenta without any knowledge if the baby really needs this treatment. However, this is an ongoing discussion among experts and doctors. There isn’t a clear agreement on whether a baby should be placed in an artificial womb or allowed to be born and connected to an artificial placenta if needed. It is difficult to decide which course of action is better, because we have no prediction models and cannot be sure which treatment the baby needs. Time and results from the different research approaches will tell.’
Professor Arens recalls a story of a baby that was born in the 22nd week of pregnancy. ‘If a baby is born so early, doctors usually can’t help it. They therefore decided to only allow the mother to say goodbye, assuming that the baby would die within a couple of hours. They forgot to inform the baby about this. It started crying and kept crying louder and louder. They decided to treat it and it turned out to be a completely healthy baby in the end. On the other hand, there are babies that are born in 31st week and don’t survive or have a lot more complications. That is the problem: we have no real way of predicting what treatment the baby needs.’

Different path
Which is precisely why the ArtPlac researchers also want to leave time to assess the newborn before deciding on the needed treatment. ‘Why submerge it in a womb if it might not be necessary?’ asks Arens. ‘Also, I believe that most parents can’t imagine having their baby placed in a plastic bag. That is why we decided to explore a completely different path and develop an artificial placenta system.’
‘This type of treatment could save 700.000 – 1.2 million newborns’
If successful, the artificial placenta could have great impact on people’s lives. ‘It has been estimated by neonatologists that this type of treatment could save 700.000 – 1.2 million newborns,’ says Arens.
The placenta will be attached to the infant through the natural passage – the umbilical vessels. Normally, the umbilical cord is cut immediately after birth and the vessels close. The UT scientists aim to keep the vessels open until it’s decided whether to connect the baby to the artificial placenta or not. The baby can then stay connected to the device for as long as needed. ‘We expect it will take one or two weeks for the lungs to develop enough for baby to breathe on its own,’ adds Stamatialis.
Fiber membranes
The device will combine lung and kidney support, therefore combining the function of an artificial lung and artificial kidney into one. ‘Both the artificial lung and artificial kidney use hollow fiber membranes for the blood oxygenation and blood purification, respectively,’ explains professor Stamatialis. ‘The difference is that in current artificial kidneys, the blood flows through the bore of the fibers, while in artificial lungs it flows around the fibers. The Artplac device will combine fiber mats for blood oxygenation and blood purification with blood flowing around the fibers. Hence, we will develop outside-in dialysis fibers at the UT.’
In fact, the majority of technological parts of the project will be developed at the UT. ‘The team of professor Stamatialis will work on the outside-in dialysis membrane development and production,’ says Arens. ‘In our group, we are in charge of development of a combined lung & kidney support device, of devices to keep umbilical cord vessels open and in-vitro testing of system and components. We will also provide technical support for the in-vivo tests on lambs, which will otherwise be done by our partner Maastricht UMC+ and the initiators of this project from Nuremberg.’

Proof of concept
The research project, which has been awarded the EIC Pathfinder grant, will last four years and should lead to a proof of concept. ‘We are working towards a lab prototype,’ explains professor Arens. ‘We will first develop the device, test it in-vitro as much as possible, and then test in-vivo on a premature lamb. Although we are not big fans of testing on animals, this step simply cannot be skipped with medical devices. At the end of this project, we therefore aim to have a proof of concept in an animal, after which it will take a few more years before the device is approved and can be used in clinic. Getting a medical device approved is very complex, but luckily our consortium has the necessary knowledge to make this happen.’